Class of 2018: Kyle Kirkpatrick helps to crack long-sought key to make quantum computing a reality
Kyle Kirkpatrick in chemistry lab

Longtime Virginia Tech chemistry professor Harry Dorn thinks he has found a magic bullet to break open the international race of quantum computing.
Dorn has graduating senior Kyle Kirkpatrick of Vienna, Virginia, to thank for much of the traction in this breakthrough, originally made by Dorn himself back in 2007. For now, quantum computing is more theory than reality, but the idea is to store data on quantum bits, which are molecules that are vastly smaller than the current transistor bits we use now to store our websites, photographs, emails, movies, and more.
But Dorn and Kirkpatrick think a lab-made single-molecule magnet with the tongue-twister name of azaheterometallofullerene or M(2)@C(79)N is the quantum bit for the job. Skipping past some really complicated science, magnets are not just tools for holding a child’s drawing to the refrigerator or the basis for MRI machines. Magnets can store data.
More so, single-molecule magnets can store vast droves of data, a 1,000-times increase in storage capacity from current transistor bytes. That’s because single molecules are tiny and can store data not only in 1s and 0s, but they also can store each of those numbers in “up” or “down” states, with multiple available combinations.
“As the user demand for greater and greater storage capacity increases, we need to figure out how to store data on a smaller scale,” said Kirkpatrick, a major in the Department of Chemistry and minor in the Academy of Integrated Science’s nanoscience program, all part of the Virginia Tech College of Science.
“The use of single-molecule magnets could significantly increase the amount of storage that is possible. Instead of having a 256-gigabyte cell phone, you could have a 256-terabyte cell phone,” Kirkpatrick said.
Computer storage won’t just undergo a revolution with quantum computing. The entire computer industry will. Dorn points to higher detailed MRI images for surgeons and satellite images for security personnel. And researchers, agencies, and companies that now rely on modern supercomputers will see even faster tools at their disposal. But all of this will take time in the lab. Several years of testing, including those involving operating temperature ranges in the extreme cold of liquid helium and liquid nitrogen, are ahead.
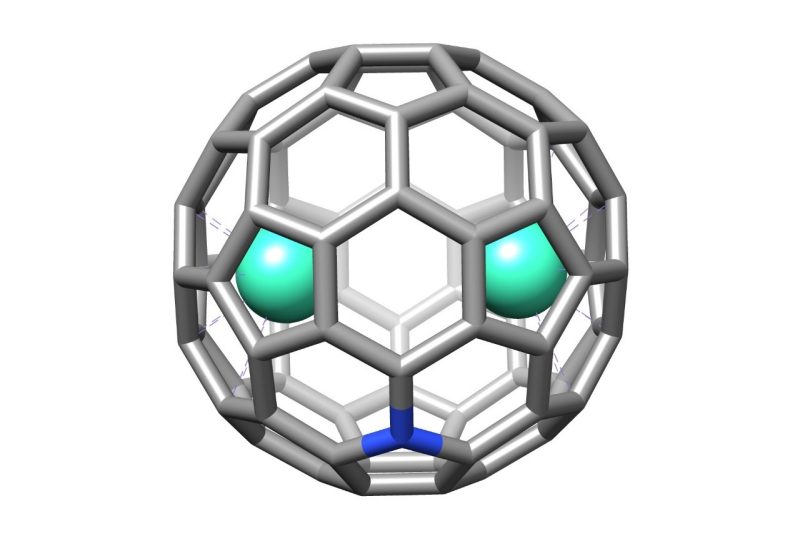
A graphic representation of the single-molecule magnet Gd(2)@C(79)N, which chemistry student Kyle Kirkpatrick was able to separate from a larger molecule.
The race to crack quantum computing is fierce, said Dorn, a professor of chemistry and an affiliated faculty member at the Virginia Tech Carilion School of Medicine and Research Institute.
“The National Science Foundation, the U.S. Army, the U.S. Navy, DARPA, and the U.S. Department of Energy are all funding quantum computing,” Dorn said. “IBM and other computer companies are heavily involved. Researchers in Japan, China, Germany, and England are involved at the best facilities in each country, with Oxford University being a hotbed for developing quantum computing.”
Dorn’s work in single-molecule magnets and molecular semiconductors began in 1999. Dorn developed in his lab a molecule known as Gd(3)N@C(80). (A basic explainer of this chemical name: The molecule is a soccer-ball-shaped cage of 80 carbon atoms and one nitrogen atom, with three atoms of the gadolinium metal atoms inside.) Virginia Tech sold rights to Danville, Virginia-based Luna Innovations.
Flashforward to 2017 and Dorn discovered that the Gd(3)N@C(80) molecules sold by Luna contained traces of an unintended byproduct. This “impurity” was the single-molecule magnet, Gd(2)@C(79)N. The difference? The “new” molecule is missing one gadolinium atom and one carbon atom is replaced with that of a nitrogen atom.
This single-molecule magnet quickly drew mass attention by quantum chemists, but it was Kirkpatrick as an undergraduate researcher who separated the Gd(2)@C(79)N. He also was able to recreate the single-molecule magnet as Tb(2)@C(79)N, using terbium (Tb) atoms instead of gadolinium (Gd). This later step is vital because as the single-molecule magnet can use different metals, it can carry the chemical name M(2)@C(79)N, where the “M” simply stands for a metal, Kirkpatrick said.
Before Kirkpatrick starts graduate school at the University of California San Diego in August, he will have co-authored five to six papers in the fields of dynamic nuclear polarization, single-molecule magnetism, and quantum computing. “No other chemistry undergraduate student has accomplished this in my laboratory during the past 43 years,” Dorn said.
He added, “Kyle already operates as an advanced graduate student, not an undergraduate. Kyle has a real zeal for science, and I predict he will soon be a superstar.”
Kirkpatrick credits chemistry faculty for pushing students such as himself into undergraduate research. He also found encouragement in the College of Science’s burgeoning nanoscience program.
“It’s one of the first programs of its kind and really prepares students on how to tackle big problems that face our generation, such as energy,” he said. “I have enjoyed the interdisciplinary nature of nanoscience and its intersections with chemistry, physics, and biology. Plus, it’s cool to tell people you are a nanoscientist.”
Related story
Researchers discover evidence to support controversial 'buckyball' formation theory