Collaborative grant takes on brain cancer cell invasion
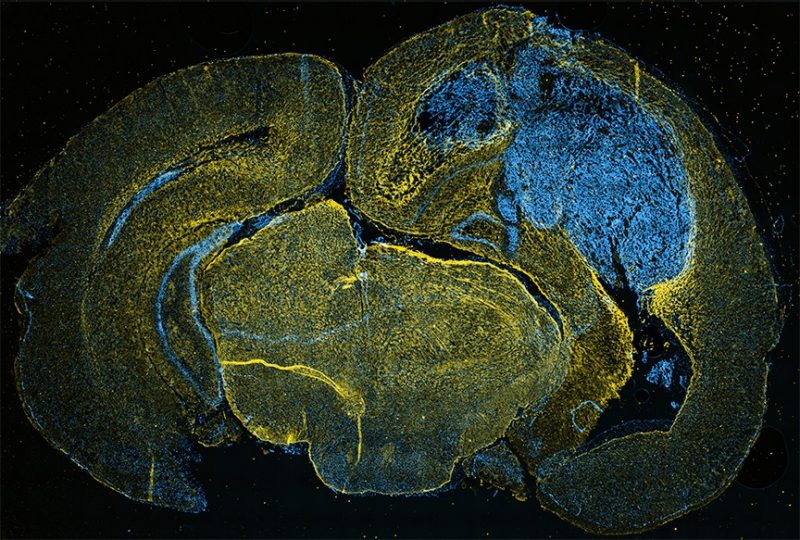
Dotting the white walls of Jennifer Munson’s lab at Virginia Tech are large bright pictures of microscopic cells and fluid.
Though their vibrant colors and swirling, abstract patterns may appear fascinating – even beautiful – to lab visitors, these snapshots of fluorescing tissue tell a darker story. Each photo is a live-image capture of cancer cells at work.
Most recognizable to the untrained eye are vivid patches of material confined to the unmistakable form of the human brain. In these images, glioma cells, or brain cancer cells, have begun to overlap with the white and gray matter of normal, healthy brain tissue.
It is precisely this type of cancer cell invasion that Munson, an assistant professor in the Department of Biomedical Engineering and Mechanics in the College of Engineering, hopes to prevent. With a recent $2.6 million grant from the National Institutes of Health, she’ll lead a multidisciplinary, multisite team of researchers in pursuit of new therapies to improve the standard of care for brain cancer patients.
Munson’s lab at Virginia Tech will collaborate with researchers at the University of Virginia and the University of Michigan for the duration of the five-year grant, which will examine the role of interstitial fluid flow in glioma cell invasion.
A relatively unexplored area of cancer research, interstitial fluid flow, or the movement of fluid around and through the three-dimensional space surrounding cells, has been shown to lead to an increase in cancer cell invasion. However, the mechanisms of how and why that happens, particularly in cases of brain cancer, are still unknown.
“The end goal is that we identify therapies that can be used against glioma,” said Munson. “And really studying interstitial fluid flow and fluid drainage in brain cancer is a completely untapped area to look for targeted therapies. So we’re taking everything that’s out there and looking at it in a new context.”
That novel approach to brain cancer research will require a creative take on teamwork, one that relies heavily on a wide scope of scientific and medical experts.
“We’re working with other engineers, MRI physicists, physician scientists, and oncologists, as well as basic scientists and chemists to try to look at something that’s totally unexplored,” said Munson.
“To do that, we need this interdisciplinary team not only to keep us clinically relevant, but also to do the best science we can do,” she said.
Because brain tumors grow within a very confined area, the tumor’s developing tissue significantly increases the pressure in the space between cells, especially when compared to the surrounding healthy tissue. Fluid drainage from those high-pressure areas afflicted with tumors to low-pressure areas of healthy tissue are the focus of the team’s research.
B.J. Purow, a professor of clinical neurology and physician scientist at the University of Virginia, will use MRI technology to image this interstitial fluid movement as the brain tumors progress and throughout different types of clinical treatment. Purow will work closely with Fred Epstein, professor and chair of biomedical engineering, and Hui Zong, an associate professor of microbiology, immunology, and cancer biology, toward these imaging efforts at UVA.
Munson’s team at Virginia Tech will develop code and mathematical algorithms to track, describe, and analyze fluid flow in the UVA team’s MRI images with hopes of correlating flow with potential new biomarkers for therapeutic targeting.
At the University of Michigan, Kathryn Luker, an associate research scientist in radiology, will use patient-derived cancer cells to genetically engineer new cells that respond to fluid flow in specific ways.
Munson will then use those cells to take live images of interstitial flow within patient-derived, tissue-engineered models of brain cancer. Rebecca Pompano, an assistant professor of chemistry at UVA, will create microfluidic devices for Munson during this phase of the grant to examine cancer cell invasion under flow. Munson plans to use these microfluidic devices to examine the effects of flow within the context of the complete tumor microenvironment.
Bethany Horton, an assistant professor of public health genomics at UVA, rounds out the comprehensive grant team and will provide statistical support.

Munson’s close work with UVA researchers comes as no coincidence. She joined the biomedical engineering faculty there in 2014 and recently moved across the commonwealth to join Virginia Tech in August 2017. Much of her research on interstitial flow and the tumor microenvironment began at UVA and will continue to progress both in Blacksburg and Charlottesville.
In addition to her collaborators at other universities, Munson will rely on the help of Virginia Tech students to work through several of the grant’s research questions. First-year biomedical engineering doctoral student Caleb Stine will analyze the images of interstitial flow from UVA. Chase Cornelison, a postdoctoral research associate in Munson’s lab, has already been examining the effects of interstitial flow on the tumor microenvironment for over a year, along with Kathryn Kingsmore, a biomedical engineering doctoral student at UVA. More students will join in Munson’s research efforts as the grant progresses.
“My approach is pretty hands-on, because you kind of have to be,” said Munson of involving students both in research and the grant process. “We’re a team, and we work together toward the mutual goals of the lab. Those are not only my goals, but also their goals. These experiences will help prepare students for whatever they want to do with their careers.”
For at least the next several years, those experiences will revolve around stopping the spread of cancer to healthy parts of the brain. Patients afflicted with glioblastoma, the most aggressive type of glioma, face an average survival time of 13 months after diagnosis. With invasion as one of its hallmarks, glioblastoma is notoriously difficult to treat, and typical standard of care therapies aim to remove the tumor bulk through intensive surgeries.
“These patients always have surgery and then they go through rounds of radiation and chemotherapy that are very aggressive,” said Munson. “But every one of these cancers recurs, so there really are no long-term survivors of this disease.”
“We need new approaches and we need new targets,” she said.
Written by Emily Roediger






.jpg.transform/m-medium/image.jpg)